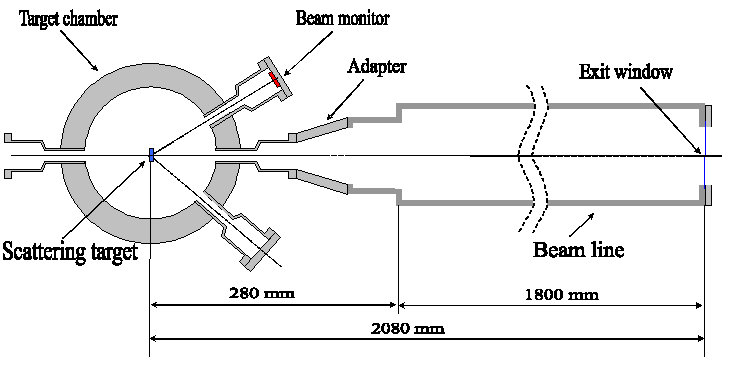








The Warsaw cyclotron facility with a horizontal beam for radiobiological studies.Joanna Czub (1), Tomasz Adamus (2), Dariusz Banaś (1), Janusz Braziewicz (1), Jarosław Choiński (5), Jan Dyczewski (2), Marian Jaskóla (4), Andrzej Korman (4), Zygmunt Szefliński (2), Andrzej Wójcik (3), (1) Institute of Physics, Świętokrzyska Academy, Kielce, Poland The understanding of radiobiological effects of charged heavy particles are of fundamental importance both for radiation protection and radiotherapy, where the number of patients treated with heavy ions is increasing [1,2]. The physical environment of the sample is the major problem in biological studies of living cells under irradiation by the uniform beam of heavy ions. The interaction of densely ionizing particles with DNA in the cell nucleus yields important information to the models describing both the track structure, the DNA-geometry as well as the effect of radiation on the cell. The knowledge of radiation-induced DNA-breaks allows, for instance, to estimate the risks from radiation exposure at low doses (e.g. during long-manned space flights). The technical realization of a particle irradiation facility with a horizontal beam-line at the HIL is now tested. In the experiment at the Warsaw Cyclotron horizontal beams of the 100-60 MeV 12C ions are extracted to the atmosphere from the vacuum tube through an exit window made from the Havar foil 2.5 mg/cm2 thick with a size of 13 x 13 mm. The Au scattering foils of different thickness 20 - 60 mg/cm2 are used to produce a uniformly distributed beam directed to exit window placed at 00 and to the beam monitor located at 150. A sketch of the horizontal beam line of the HIL is shown in Fig.1.The uniform spatial distribution of the beam intensity at the exit window is assured by the small-angle multiple Rutherford scattering events inside the foil. The cell containers fastened to an X-Y sliding table (Fig. 2) is moved along the X and Y axes in order to ensure a uniform dose distribution at the irradiated target. Horizontal beam lineWith the Warsaw cyclotron almost all types of ions with high linear energy transfer (LET) can be accelerated. The beams are transported by a horizontal line with conventional beam tuning components including a fast mechanical beam shutter, which can be activated by the irradiation control system. The beams leave the vacuum tube through an exit window with a size 13*13mm sealed by a 2.5 mg/cm2 thick Havar foil. The
thickness of all materials passed by the beams is controlled to
assure the desirable energy, i.e. in the region of the Bragg peak, at
our radiobiological experiments. Finally, the energy loss in the
irradiated cells can be varied from ~4 keV/μm
to ~50 keV/μm. Fig. 1. A Sketch of the set up for cell irradiation at Warsaw cyclotron Beam spreadingTo achieve a homogeneous radiation field outside the exit window the beam is spread out using Au foils of appropriate thickness. For different beam energies and types of ions a suitable Au-foil can be rotated into the beam by a foil-changer wheel. Test measurements and Monte-Carlo calculations of the beam widening show that for a 100 MeV 12C ions a Au foil with a thickness of 40 mg/cm2 is adequate to assure the beam homogeneity better than 5% across the exit window 13*13 mm (see Fig. 3).
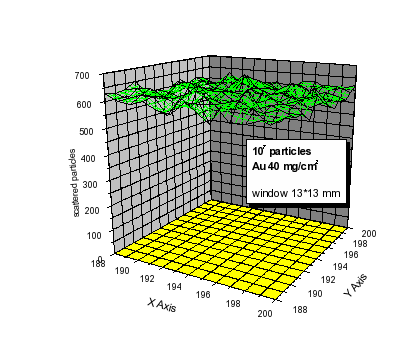 The Monte
Carlo method is adopted to simulate the beam intensity at real
experimental conditions, i.e. passing through Au-, Havar-, air- and
Mylar-foils. The beam homogeneity spreaded on the irradiated
biological area window is better than 5% (see Fig. 3).
Fig.3 Two-dimensional plot of the
number of particles at the irradiated target.
Beam monitoringThe intensity of the beam scattered at the gold scattering foil is measured by a barrier silicon detector placed at 150. To reduce the count rate, a collimator with 2mm diameter is placed in front of the detector. The detector is placed at a distance of 125mm from the center of the scattering chamber, where the scattering foil is mounted. Appropriate Monte-Carlo multiscattering simulations were performed to correlate the intensity of the scattered particles at the RBS detector and the spatial distribution at the target position. The intensity and the distribution of the scattered beam is measured
at the petri dish position in off-line measured by x-ray films.
Fig. 4. The result of irradiation of the X-ray film irradiated with doses 4.7 Gy (left), 9.5 Gy (center) and 18.9 Gy (right). Simultaneously,
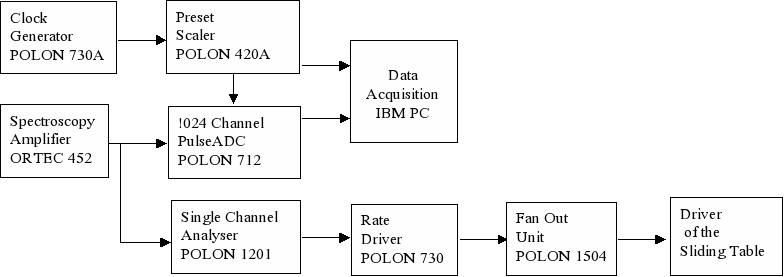
the data acquisition
system is registering time dependence of the beam intensity as well
as the energy spectrum of the scattered beam. A selected number of
counted particles initiates the programmed shift of the sliding table
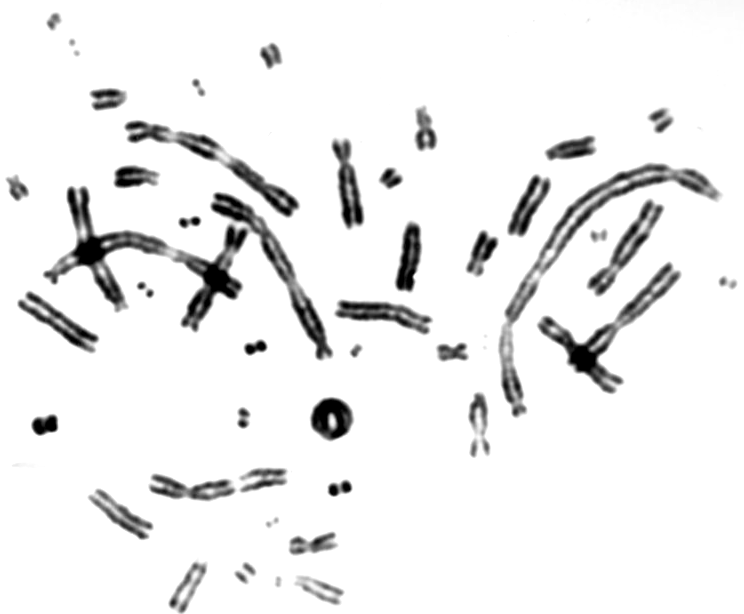
with the sample. Fig. 5. The scheme of the control system of the irradiation. In order to perform uniform irradiation of biological samples, which are spread over 6 cm in diameter, the moving sample is scanned by the beam passively smeared by the scattering foil. The required flux of the particles 107/ s of 12C at incident energy 100 MeV corresponds to the dose rate 10 Gy/min. The scanning in the horizontal and vertical direction is performed to obtain a homogeneity of the beam in the order of <5%. The dose determination based on the counting of ions scattered at 150 leads to the determination of number of particles reaching the sample. To verify the dose a set of thermoluminescence detectors, as well as an X-ray film are irradiated. The results of irradiation of the X-ray film irradiated with doses 0.25 Gy, 0.5 Gy, and 1.0 Gy respectively are shown in Fig. 5. First experimentV79 Chinese hamster fibroblasts were grown on thin foils for 24 hours, transported to the Heavy Ion Laboratory on ice and irradiated for different time periods in order to determine the optimal doses and exposure times that yield enough mitotic cells for scoring of chromosomal aberrations. Following irradiation the cells were transported on ice back to the laboratory where they were incubated at 37oC for 16 hours. Colcemid was added for the last 3 hours in order to block cells in mitosis. Cells were harvested as described elsewhere and aberrations were scored under a light microscope [3]. An example of a cell with chromosomal aberrations is shown in figure 6. A good yield of mitotic cells was observed after exposure times of 10-15 minutes and doses of 0.5-1 Gy. These exposure conditions will be used in future experiments.
Fig 6. An example of a mitotic cell with chromosomal aberrations. References: |